1. Biochemistry of Uric Acid
Uric acid is a byproduct of purine metabolism. Purines are nitrogen-containing compounds that are broken down in the liver to produce uric acid, a waste product. Uric acid then enters the bloodstream, and around 70% of it is filtered by the kidneys and expelled through urine. The remaining portion is eliminated through the gastrointestinal tract.
Key Concepts in Uric Acid Formation:
- Purine Metabolism: Purines are converted into uric acid in a process involving the enzyme xanthine oxidase.
- Solubility Limit: Uric acid has limited solubility in blood and urine; when concentrations exceed its solubility threshold, it becomes prone to crystallization.
- Normal Range vs. Hyperuricemia: Typically, blood uric acid levels between 3.5-7.2 mg/dL are considered normal. Levels exceeding this range lead to hyperuricemia, where the risk of crystal formation is high.
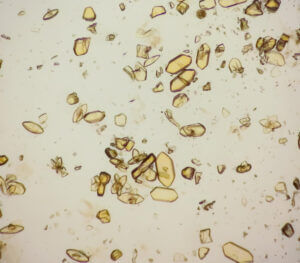
2. Mechanism of Uric Acid Crystal Formation
Supersaturation: Uric acid crystals form when blood or urine reaches a state of supersaturation. When uric acid concentration exceeds its solubility threshold (around 6.8 mg/dL in blood), the excess precipitates into solid crystals. Factors like pH, temperature, and hydration levels significantly influence solubility.
- Temperature and Solubility: Uric acid has lower solubility at cooler temperatures, which is why crystals often form in peripheral joints like the toes, where temperatures are naturally lower.
- pH Dependence: Uric acid solubility increases at higher (more alkaline) pH levels. In acidic environments (such as in dehydrated individuals), uric acid is more likely to form crystals.
Crystallization Process:
- Nucleation: Tiny clusters of uric acid molecules (called nuclei) form when uric acid concentration is high. These clusters provide a base for further growth.
- Crystal Growth: Additional uric acid molecules adhere to the nuclei, leading to the growth of solid, needle-like crystals.
- Aggregation: Crystals combine into clusters that settle in tissues, often triggering inflammation.
3. Factors Contributing to Uric Acid Crystal Formation
Several factors can push the body toward a state of uric acid supersaturation, making crystal formation more likely:
- Diet and Purine Intake: Diets rich in purines (found in red meat, shellfish, and certain fish) can significantly increase uric acid production, raising blood uric acid levels.
- Genetic Predisposition: Some individuals have genetic variations that affect uric acid metabolism, making them more susceptible to high levels and crystal formation.
- Kidney Function: The kidneys are responsible for excreting uric acid, and impaired kidney function can lead to its buildup in the bloodstream, increasing the risk of crystal formation.
- Dehydration: Insufficient hydration concentrates uric acid in the blood, reducing its solubility and promoting crystallization.
- Body Weight and Metabolic Syndrome: Being overweight or having metabolic syndrome increases uric acid production and impairs the body’s ability to excrete it, contributing to crystal formation.
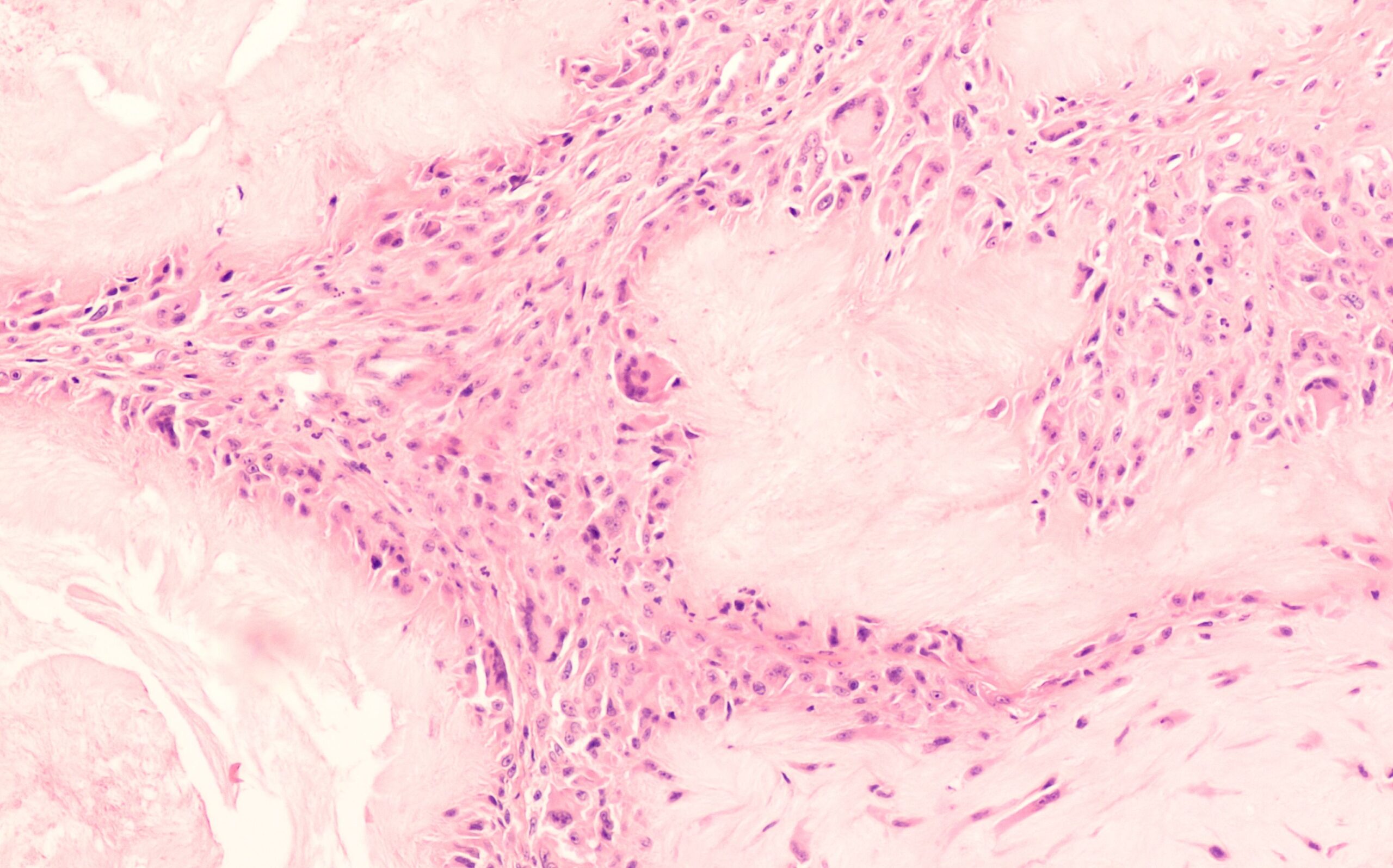
4. Uric Acid Crystal Impact on the Body
Once formed, uric acid crystals can cause significant health issues:
- Gout: The most well-known condition related to uric acid crystals, gout occurs when crystals settle in joints, causing sudden, severe pain and inflammation. This is triggered as the immune system attempts to “clean up” the crystals, leading to inflammation.
- Kidney Stones: Uric acid crystals in the kidneys can aggregate to form stones, which may obstruct the urinary tract and cause intense pain. Kidney stones can be recurrent if uric acid levels aren’t managed.
- Tophi: In chronic cases, crystals can build up under the skin, forming lumps called tophi. Tophi can become inflamed and painful and may cause deformities if left untreated.
5. Managing Uric Acid Crystal Formation
Given the chemistry behind uric acid crystals, managing their formation is a combination of dietary, lifestyle, and sometimes medical strategies:
- Alkaline Diets and Hydration: Consuming more alkaline-forming foods (like vegetables and some fruits) and staying well-hydrated can raise the pH of blood and urine, reducing the likelihood of crystal formation.
- Medications: Medications like allopurinol inhibit xanthine oxidase, the enzyme involved in uric acid production, effectively lowering uric acid levels.
- Low-Purine Diet: Avoiding foods high in purines, such as organ meats, shellfish, and some fish, can reduce uric acid production.
- Supplements: Certain supplements, like Yuri Care Uric Acid Cleanse, may support the body’s natural uric acid elimination processes.
Conclusion
Uric acid crystal formation is a complex biochemical process influenced by diet, genetics, hydration, and kidney function. Understanding the science behind uric acid crystals sheds light on how diet and lifestyle adjustments can prevent crystal accumulation, reducing the risk of painful conditions like gout and kidney stones. Regular monitoring and mindful dietary choices are critical for those at risk, as is staying hydrated and maintaining healthy kidney function.